OP.CALIFE® CANH NIEN AN TABLET
FORMULA:
For 1 film coated tablet
Active pharmaceutical ingredients:
Cortex Radicis Paeoniae suffruticosae 33 mg
Radix Fallopiae multiflorae 50 mg
Fructus Schisandrae chinensis 50 mg
Rhizoma Alismatis 50 mg
Radix Rehmanniae glutinosae 50 mg
Radix Scrophulariae 50 mg
Radix Ophiopogonis japonici 50 mg
Radix Rehmanniae glutinosae praeparata 50 mg
Ramulus cum Unco Uncariae 100 mg
Caulis Fallopiae multiflorae 100 mg
Poria 100 mg
Rhizoma Curculiginis 100 mg
Magnetitum 100 mg
Concha Margaritifera 100 mg
Fructus Tritici Levis 100 mg
Excipients: Lactose, maize starch, calcium carbonate, povidone, microcrystal cellulose, sodium starch glycolate, talc powder, magnesium stearate, sodium benzoate, chitosan, amaranth red, ponceau 4R red lake.
DOSAGE FORM:
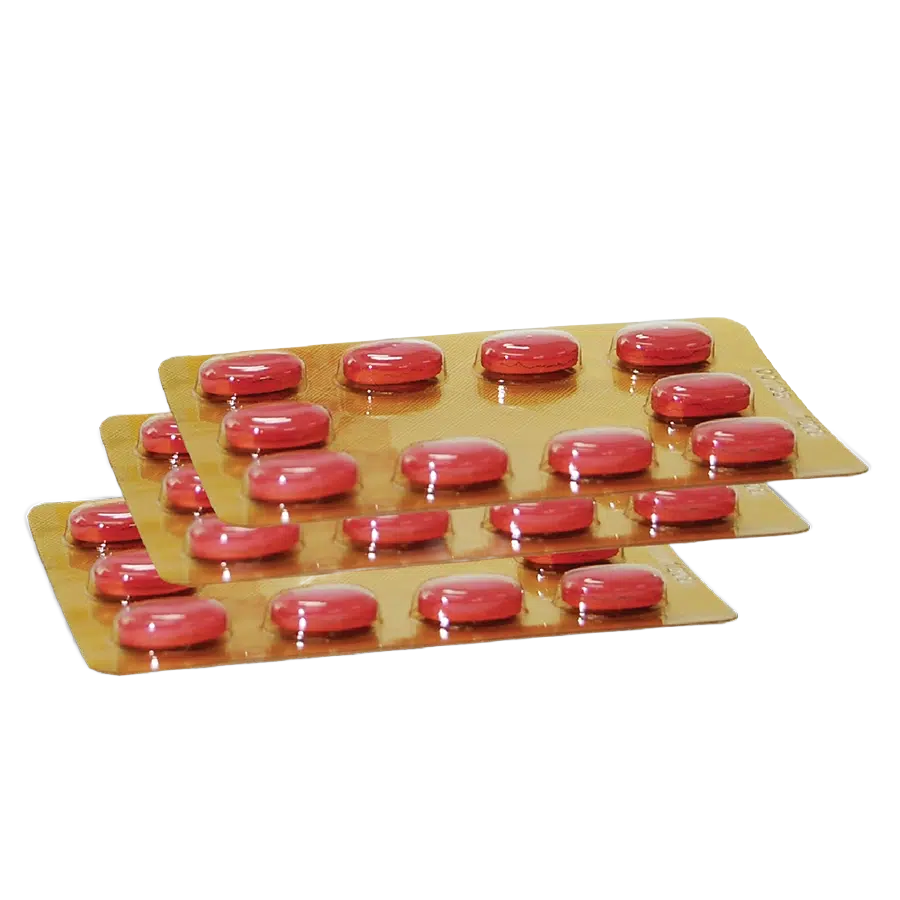
Film coated tablets are elongated, purple-red, brown inside, characteristic aroma, bitter taste.
ACTIONS:
Nourishing yin and purging fire.
INDICATIONS:
Used in pre-menopause period with the following symptoms: Hot flashes, sweating, vertigo, tinnitus, restlessness, anxiety, insomnia, unstable blood pressure.
This medicine can be used in conjunction with Hormone Replacement Therapy on doctor’s prescription.
DOSAGE AND ADMINISTRATION:
Take orally 3 tablets each time, 3 times per day until the symptoms are under the control. Maintenance dosage: 2 – 3 tablets per time, 2 times per day. Take orally after meal. It is recommended to be taken with weak green tea.
Or follow your doctor’s instructions.
CONTRAINDICATIONS:
People with yang deficiency, cold syndrome.
Pregnant women, lactating women.
People with diarrhea.
People with hypersensitivity to any ingredient of the medicine.
WARNINGS AND PRECAUTIONS:
Not reported yet.
PREGNANT WOMEN AND LACTATING WOMEN:
Do not use.
DRIVING AND OPERATING MACHINE ABILITY:
No effect.
DRUGS INTERACTIONS, DRUGS INCOMPATIBILITIES:
a) Drugs interactions:
Avoid of food irritating the stomach such as coffee, alcoholic drinks, strong tea, etc.
Restrict consuming too much food containing carbohydrate and high molecular lipid.
Not reported yet.
b) Drugs incompatibilities:
Because there have not been any studies about incompatibilities of the drug, do not mix with other drugs.
UNDESIRABLE EFFECTS:
Not reported yet.
Inform the doctor or the pharmacist immediately if any undesirable effect occurs while taking this medicine.
OVERDOSE AND TREATMENT OF OVERDOSE:
There has not been any information about overdose, thus do not use overdose.
PRESENTATION:
Box of 5 blisters x 10 tablets.
STORAGE:
In a dry place, do not store above 30°C.
SHELF LIFE:
36 months from manufacturing date.
SPECIFICATION:
Manufacturer’s.
MANUFACTURER RESPONSIBLE FOR THE GOODS:
OPC Pharmaceutical Joint Stock Company (1017 Hong Bang, Ward 12, District 6, Ho Chi Minh City).
MADE IN VIETNAM.