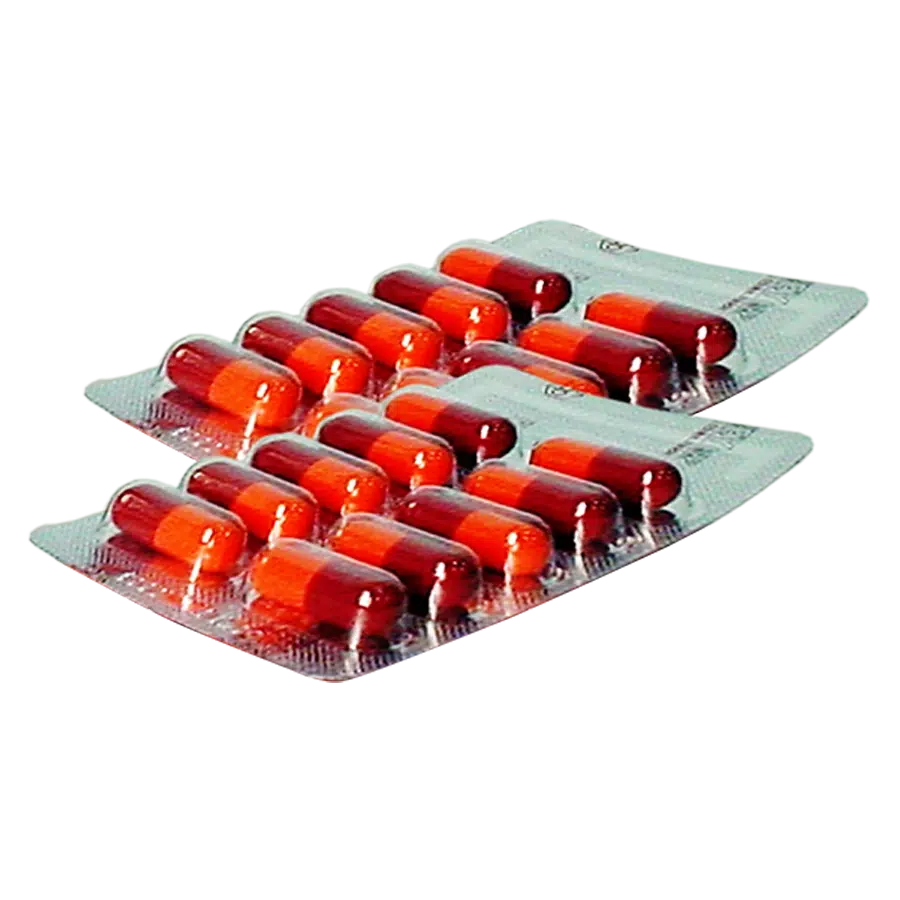
KIDNEYCAP® Capsule of Eight Ingredients or Kidney-Yang Replenishment
FORMULA:
For 1 capsule
Active pharmaceutical ingredients:
Extract on dry basis 100.00 mg
Equivalent to:
Radix Rehmanniae glutinosae praeparata 262.50 mg
Tuber Dioscoreae persimilis 189.82 mg
Fructus Corni officinalis 220.00 mg
Cortex Radicis Paeoniae suffruticosae 80.08 mg
Rhizoma Alismatis 162.50 mg
Radix Aconiti lateralis praeparata 55.00 mg
Poria 125.00 mg
Powder mixture 170.10 mg
Equivalent to:
Tuber Dioscoreae persimilis 50.18 mg
Poria 37.50 mg
Cortex Radicis Paeoniae suffruticosae 82.42 mg
Cortex Cinnamomi 55.00 mg
Excipients: Maize starch, magnesium carbonate light, talc powder, magnesium stearate, hard capsule
DOSAGE FORM:
Hard capsule: shiny, not sticky powder outside. To has brownish powder inside, characteristic odor, a slightly vinegar taste.
INDICATIONS:
Deficiency of the kidney-yang, headache, tinnitus, back aches, knees tiredness, cold lower half of body, night urination, cold perspiration.
DOSAGE AND ADMINISTRATION:
Take orally 2 capsules each time, 3 times per day. Take orally before meals.
Or follow doctor’s instructions.
CONTRAINDICATIONS:
Pregnant women, person suffering from newly fever, constipation, children below 15 years old, people with diabetes insipidus, hot property, thirst desire, deficiency of blood.
People with hypersensitivity to any ingredient of the medicine
taking this medicine.
WARNINGS AND PRECAUTIONS:
Do not exceed the recommended dose. Diabetics should follow doctor’s instruction while using the medicine. Patients with heart failure should take the medicine with caution.
PREGNANT WOMEN AND LACTATING WOMEN:
Do not take.
DRIVING AND OPERATING MACHINE ABILITY:
No effect.
DRUGS INTERACTIONS, DRUGS INCOMPATIBILITIES:
a) Drugs interactions:
Not reported yet.
b) Drugs incompatibilities:
Because there has not been any studies about incompatibilities of the drug, do not mix with other drugs.
UNDESIRABLE EFFECTS:
Not reported yet.
Inform the doctor or the pharmacist immediately if any undesirable effect occurs while taking this medicine.
OVERDOSE AND TREATMENT OF OVERDOSE:
There has not been any information of overdose; thus do not use overdose.
PRESENTATION:
Box of 5 blisters of 10 capsules.
STORAGE:
In a dry place, do not store above 30°C.
SHELF LIFE:
36 months from manufacturing date.
SPECIFICATION:
Manufacturer’s.
MANUFACTURER RESPONSIBLE FOR THE GOODS:
OPC Pharmaceutical Joint Stock Company (1017 Hong Bang, Ward 12, District 6, Ho Chi Minh City).
MADE IN VIETNAM.