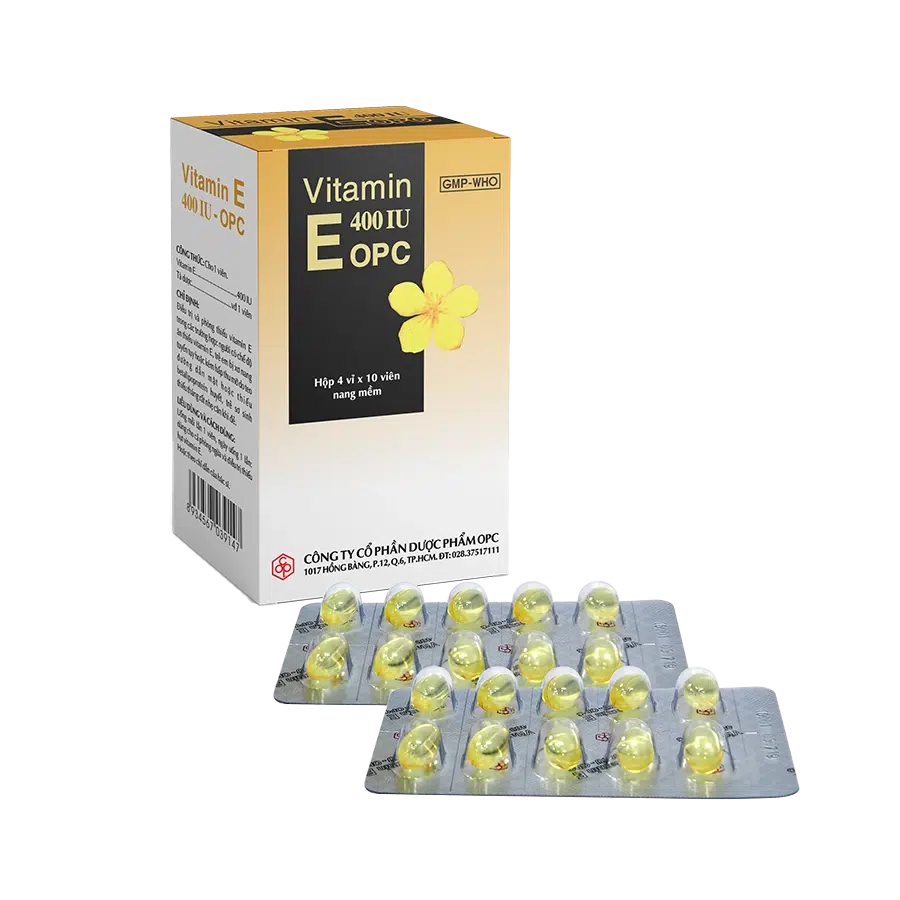
VITAMIN E 400 IU – OPC
FORMULA:
Vitamin E 400 IU
Excipients (gelatin, glycerin, nipasol M, purified water) s.q.f 1 soft capsule
PHARMACODYNAMICS:
Vitamin E helps remove symptoms of Vitamin E deficiency and is used as an antioxidant through such mechanism as: prevent the oxidation of necessary ingredients in cells, prevent the formation of poisonous products like peroxidation products created by the oxidation of polyunsaturated fatty acids, react with free radicals, which are the cause of oxidative damage to cell membranes, without the formation of another free radical in the process.
PHARMACOKINETICS:
Absorption of Vitamin E from the gastrointestinal tract is dependent on the presence of bile and on normal pancreatic function. The amount of Vitamin E absorbed decreases as the dose is increased. Vitamin E enters the blood via the chylomicrons in the lymph and is widely distributed to all tissues and stored in adipose tissue. Some Vitamin E is metabolized in the liver to glucuronides of tocopheronic acid and its gamma-lacton, and then excreted in the urine, but most of a dose is slowly excreted in the bile. Vitamin E appears in breast milk but is poorly transferred across the placenta.
INDICATIONS:
Treat vitamin E deficiency in cases of insufficient intakes of vitamin E, children with cystic fibrosis of pancreas or fat malabsorption because of biliary malabsorption or abetalipoproteinaemia, very low birth weight premature infants; myopathic or neurological diseases such as reduction of reflex, abnormal carriage, tardive dyskinesia, ophthalmoplegia, retinal pigmentation and axon degeneration.
Prevent the oxidation in combining with vitamin C, vitamin A and selenium.
Supportively treat fatty liver, hypercholesterolemia, infertility, low sperm count.
Prevent vitamin E deficiency.
DOSAGE AND ADMINISTRATION:
Take orally 1 soft-gel daily, for both prevention and treatment of Vitamin E deficiency or as directed by the physician.
CONTRAINDICATIONS:
People with hypersensitivity to any ingredient of the medicine.
PRECAUTIONS:
Be cautious when using the medicine for patients using anticoagulant medicines.
PREGNANT WOMEN AND LACTATING WOMEN:
During pregnancy: Neither deficiency nor excess of Vitamin E has been associated with maternal or fetal complications during pregnancy. In well-nourished women, adequate Vitamin E is consumed in the diet and supplementation is not required. If dietary intake is poor, supplementation up to the recommended daily allowance (RDA) for pregnancy is recommended.
During lactation: Vitamin E is excreted into breast milk. Human milk is more than five times richer in Vitamin E than cows milk and is more effective in maintaining adequate serum Vitamin E up to 1 year of age. The RDA of Vitamin E during lactation is 12 mg. Maternal supplementation is recommended only if the diet does not provide sufficient Vitamin E to meet the RDA.
DRIVING AND OPERATING MACHINE ABILITY:
No effect.
SIDE EFFECTS:
Vitamin E is usually well tolerated. Large dose may cause diarrhea, abdominal pain, and other gastrointestinal disturbances and also cause fatigue, weakness. Some transient undesirable effects may happen because the medicine contain 30% of ethanol such as impaired vision, impaired coordination of movement, rapid heartbeat, emotional disorder, transient dementia, nausea, vomiting, etc.
Inform the physician immediately if any side effect occurs while using the medicine.
DRUGS INTERACTIONS:
Vitamin E is antagonistic with Vitamin K, therefore increase the coagulation time. Vitamin E concentration decreases in people suffered from drug-induced malabsorption such as malabsorption as a result of cholestyramin use.
Vitamin E may increase the effects of oral anticoagulants, warfarin and aspirin.
OVERDOSE AND TREATMENT OF OVERDOSE:
Not found.
DOSAGE FORM AND PRESENTATION:
Soft capsule. Box of 4 blisters of 10 capsules.
SHELF LIFE:
36 months from manufacturing date.
STORAGE:
In a cool and dry place, temperature below 30°C, protected from light.
SPECIFICATION:
Manufacturer’s.
MANUFACTURER RESPONSIBLE FOR THE GOODS:
OPC Pharmaceutical Joint Stock Company (1017 Hong Bang, Ward 12, District 6, Ho Chi Minh City).
MADE IN VIETNAM.