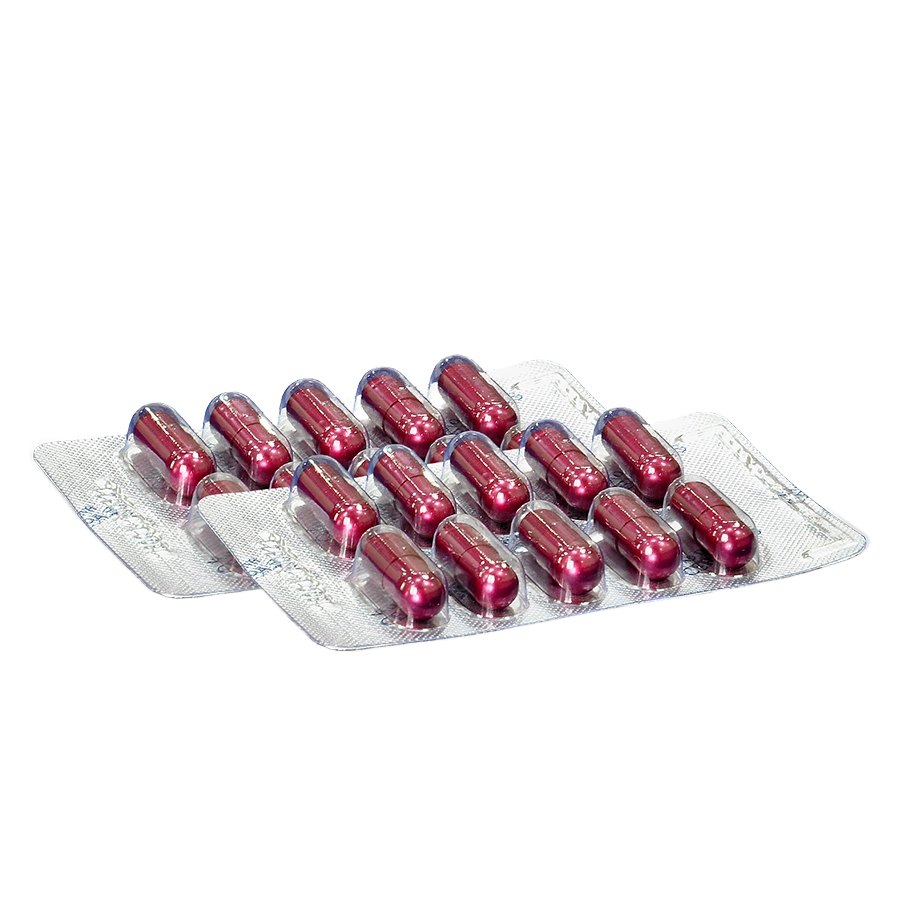
NAO DAC SINH CERINPAS®
FORMULA:
For 1 capsule
– Active pharmaceutical ingredients:
Fine powder of Sanchi 234 mg
Equivalent to:
Radix Panasis notoginseng 234 mg
Viscous extract on dry basis 140 mg
Equivalent to:
Flos Carthami 272 mg
Fructus Crataegi 470 mg
Rhizoma Chuanxiong .234 mg
Dry extract of Kudzu 75 mg
Equivalent to:
Radix Puerariae lobatae 784 mg
– Excipients:
Maize starch, lactose, magnesium stearate, sodium benzoate, hard capsule.
DOSAGE FORM:
Hard capsules.
Product descriptions: Hard capsules size 0, brownish-purple with silver lustre, glossy. To has brownish-yellow powder inside, characteristic herbal aroma, slightly bitter taste.
ACTIONS:
To activate blood, resolve stasis, unblock the meridian and harmonize collaterals.
INDICATIONS:
Dizziness, stroke caused by static blood obstructing collaterals, manifested as paralyzed limbs, sluggish speech, dizziness and vertigo. Cerebral arteriosclerosis, ischemic stroke, cerebral haemorrhage sequela with the symptoms described above.
DOSAGE AND ADMINISTRATION:
Adult: 2 capsules each time, 3 times per day. Take orally after meals with warm water.
Or follow your doctor’s instructions.
CONTRAINDICATIONS:
Pregnant women and lactating women.
People with acute brain hemorrhage.
People with peptic ulcers, menorrhagia, influenza.
People with acute myocardial infarction.
People with hypersensitivity to any ingredient of the medicine.
WARNINGS AND PRECAUTIONS:
People with a history of stomach pain.
Dosage must be adjusted appropriately in people who need to monitor clotting factors, people who are being treated cardiovascular disease, blood pressure.
A periodic health examination should be made when this medicine has been used for a long time.
After taking this medicine for about 2 weeks, see your doctor if your symptoms don’t improve.
PREGNANT WOMEN AND LACTATING WOMEN:
Do not take.
DRIVING AND OPERATING MACHINE ABILITY:
Not reported yet.
DRUGS INTERACTIONS, DRUGS INCOMPATIBILITIES:
a) Drugs interactions:
The co-administration with anticoagulants must be monitored because this medicine has platelet aggregation inhibitory effects.
This medicine should not be used in combination with hemostatic drugs.
This medicine increases cerebral and coronary blood flow; reduces blood lipid levels and blood viscosity; prevents blood clots; reduces blood pressure, so when used in combination with other medicines, the dose of this medicine should be adjusted appropriately in each patient. Before using this medicine, consult your doctor or pharmacist when used in combination with other medicines.
Reduce salt and sugar in your diet when using this medicine. Avoid spicy, cold and greasy food. Don’t smoke.
b) Drugs incompatibilities:
Because there have not been any studies about incompatibilities of the drug, do not mix with other drugs.
UNDESIRABLE EFFECTS:
Not reported yet.
Inform the doctor or the pharmacist immediately if any undesirable effect occurs while taking this medicine.
OVERDOSE AND TREATMENT OF OVERDOSE:
If any uncomfortable symptoms occur due to overdose, stop taking this medicine immediately, see your doctor for examination and treatment.
PRESENTATION:
Box of 5 blisters × 10 capsules.
STORAGE:
In a dry place, do not store above 30 oC.
SHELF LIFE:
36 months from manufacturing date.
SPECIFICATION:
Manufacturer’s.
MANUFACTURER RESPONSIBLE FOR THE GOODS:
OPC Pharmaceutical Joint Stock Company (1017 Hong Bang Ward 12 District 6 Ho Chi Minh City).
MADE IN VIETNAM.