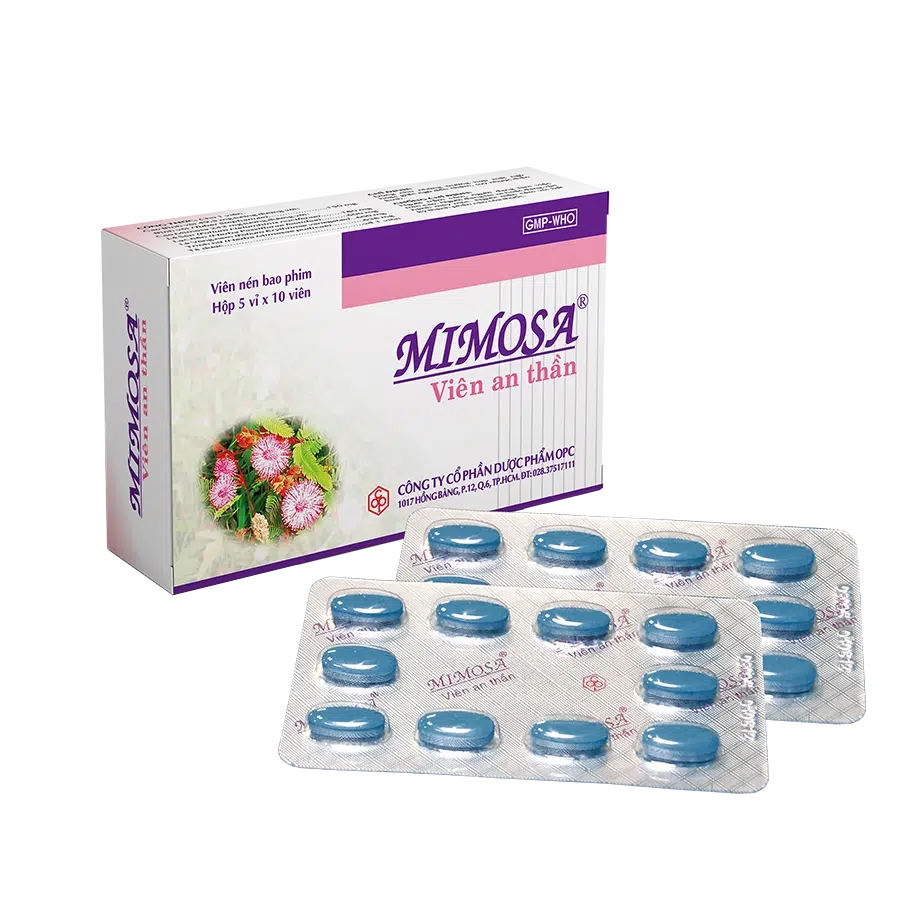
MIMOSA® SEDATIVE TABLET
FORMULA:
For 1 tablet
Active pharmaceutical ingredients:
Tuber Stephaniae extract on dry basis 49.5 mg
Equivalent to:
Tuber Stephaniae 150 mg
Extract on dry basic 242 mg
Equivalent to:
Folium Nelumbinis nuciferae 180 mg
Herba Passiflorae foetidae 600 mg
Folium Erythrinae variegatae 600 mg
Herba Mimosae pudicae 638 mg
Excipients: Lactose, Maize starch, povidone, cellulose powder, sodium starch glycolat, talc powder, magnesi stearate, indigocarmin blue lake, chitofilm powder, sodium benzoate
DOSAGE FORM:
Film-coated tablet: blue film-coated tablet, brown inner core and bitter taste.
INDICATIONS:
Insomnia or sleeplessness, mental depression.
DOSAGE AND ADMINISTRATION:
– Adults: take orally 1 – 2 tablets in 30 – 60 minutes prior to bedtime.
Children 5 – 15 years of age: half of adult’s dose.
Or follow your doctor’s instructions.
CONTRAINDICATIONS:
People with depression, people while working at high places.
People while driving vehicles or operating machine.
People with hypersensitivity to any ingredient of the medicine.
WARNINGS AND PRECAUTIONS:
Do not exceed recommended dose.
PREGNANT WOMEN AND LACTATING WOMEN:
Not reported yet, only take this medicine when the benefits are greater than the risks.
DRIVING AND OPERATING MACHINE ABILITY:
Do not take this medicine while driving vehicles or operating machine.
DRUGS INTERACTIONS, DRUGS INCOMPATIBILITIES:
a) Drugs interactions:
Not reported yet.
b) Drugs incompatibilities:
Because there have not been any studies about incompatibilities of the drug, do not mix with other drugs.
UNDESIRABLE EFFECTS:
Not reported yet.
Inform the doctor or the pharmacist immediately if any undesirable effect occurs while taking this medicine.
OVERDOSE AND TREATMENT OF OVERDOSE:
There has not been any information about overdose, thus do not use overdose.
PRESENTATION:
Box of 5 blisters of 10 tablets.
STORAGE:
In a dry place, do not store above 30°C, protected from light.
SHELF LIFE:
36 months from manufacturing date.
SPECIFICATION:
Manufacturer’s.
MANUFACTURER RESPONSIBLE FOR THE GOODS:
OPC Pharmaceutical Joint Stock Company (1017 Hong Bang, Ward 12, District 6, Ho Chi Minh City).
MADE IN VIETNAM.